Fruit and vegetable batteries
Fruit And Vegetable Batteries. The voltage varied over time for all test items. How to make a battery out of pennies. Produced which in turn allows for an electrical current. The zinc is in the galvanization on the nails and the pennies are actually copper-plated zinc.

If the fruitvegetable skin is tough use the plastic knife to make an incision to insert the penny. Have you ever wondered how a clock will run off of a lemon. Fruit and Vegetable Batteries By Catherine Clem and Kelly Flora-Brownell Results. Citrus fruits tend to work well- lemons limes grapefruits and other acidic fruits and vegetables. This site is dedicated to such curiosities. It increased for the apple and the potato.
To be more specific it is dedicated to finding out how fruits and vegetables will power small devices such as clocks or calculator.
How can a lemon run a clock. Fruit and Vegetable Batteries. To be more specific it is dedicated to finding out how fruits and vegetables will power small devices such as clocks or calculator. How can a lemon run a clock. Yes you can actually use fruits and vegetables as part of an electric power source. Produced which in turn allows for an electrical current.
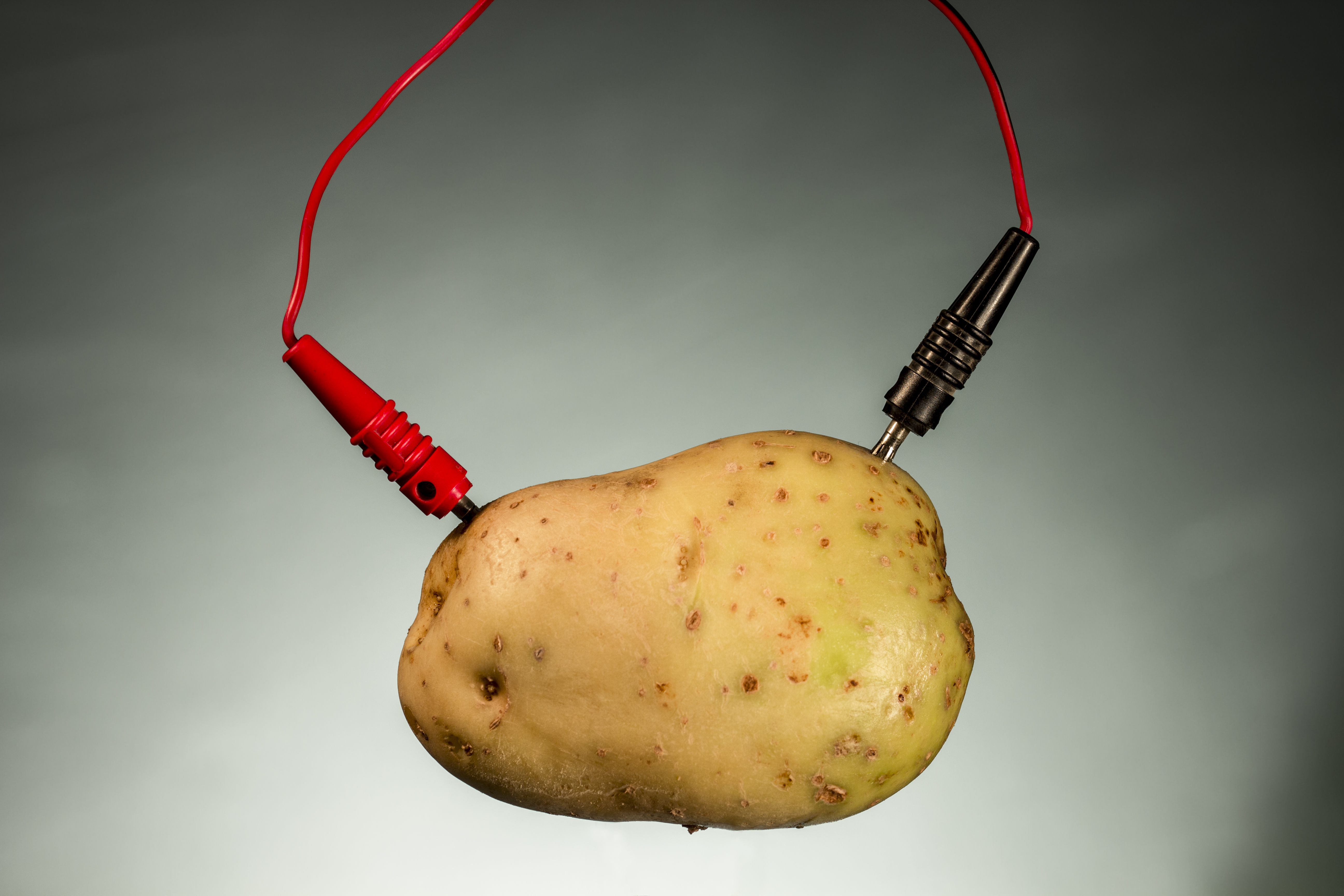
Batteries are device that store chemical energy and convert it to electrical energy so using fruit as battery acts like a wet cell that consists of a negative and positive electrode with an. Many pupils will incorrectly think that the electricity is somehow contained within the fruitvegetable. How can a lemon run a clock. It decreased for the onion mango lemon and turnip. Create a fruitvegetable battery by poking one nail into the one end of the fruitvegetable and inserting a penny into the other end of the same fruitvegetable.
 Source: youtube.com
Source: youtube.com
To be more specific it is dedicated to finding out how fruits and vegetables will power small devices such as clocks or calculator. Batteries are comprised of two different metals suspended in an acidic solution. Show full abstract fruit and vegetable waste can be used as a renewable alternative energy source in the form of bio -batteries as a substitute for conventional batteries. Powered by Create your own unique website with customizable templates. Batteries power many things around you including cell phones wireless video.
 Source: education.com
Source: education.com
Students may insert a small strip of zinc and a small strip of copper into the fruit leaving a gap of about 5cm between the two and making sure they do not come into contact with one another. Fruit and Vegetable Batteries. The voltage is a function or the metals chemical reaction to the electrolyte and can be over 1-2 volts but may be producing a very small current microamps for example. To be more specific it is dedicated to finding out how fruits and vegetables will power small devices such as clocks or calculator. The acid comes from the citric acid inside each lemon.
 Source: thoughtco.com
Source: thoughtco.com
This site is dedicated to such curiosities. How To Charge Your iPod Or iPhone With Free Energy. The voltage varied over time for all test items. Although not strictly required an understanding of some of the key terms and concepts. How can a lemon run a clock.
 Source: sciencewithkids.com
Source: sciencewithkids.com
How to make a battery out of dirt. This site is about electricity. In this simple experiment we will be creating our own battery with the use of citrus fruits with a power that is strong enough to make a small bulb light up. It increased for the apple and the potato. The fruits was cut into half and the copper and zinc metal was inserted into the fruits.

Rpedneka - A veggie or fruit based battery will generate an electro-potential voltage and current when electrodes of differing material are used. A fruit battery is an example of electrochemistry which forms the basis of all batteries. In this simple experiment we will be creating our own battery with the use of citrus fruits with a power that is strong enough to make a small bulb light up. Batteries are device that store chemical energy and convert it to electrical energy so using fruit as battery acts like a wet cell that consists of a negative and positive electrode with an. Batteries are device that store chemical energy and convert it to electrical energy so using fruit as battery acts like a wet cell that consists of a negative and positive electrode with an electrolyte which conducts ions also copper and zinc metals acts as electrodes whilecitric acid of the fruit is the electrolyte.
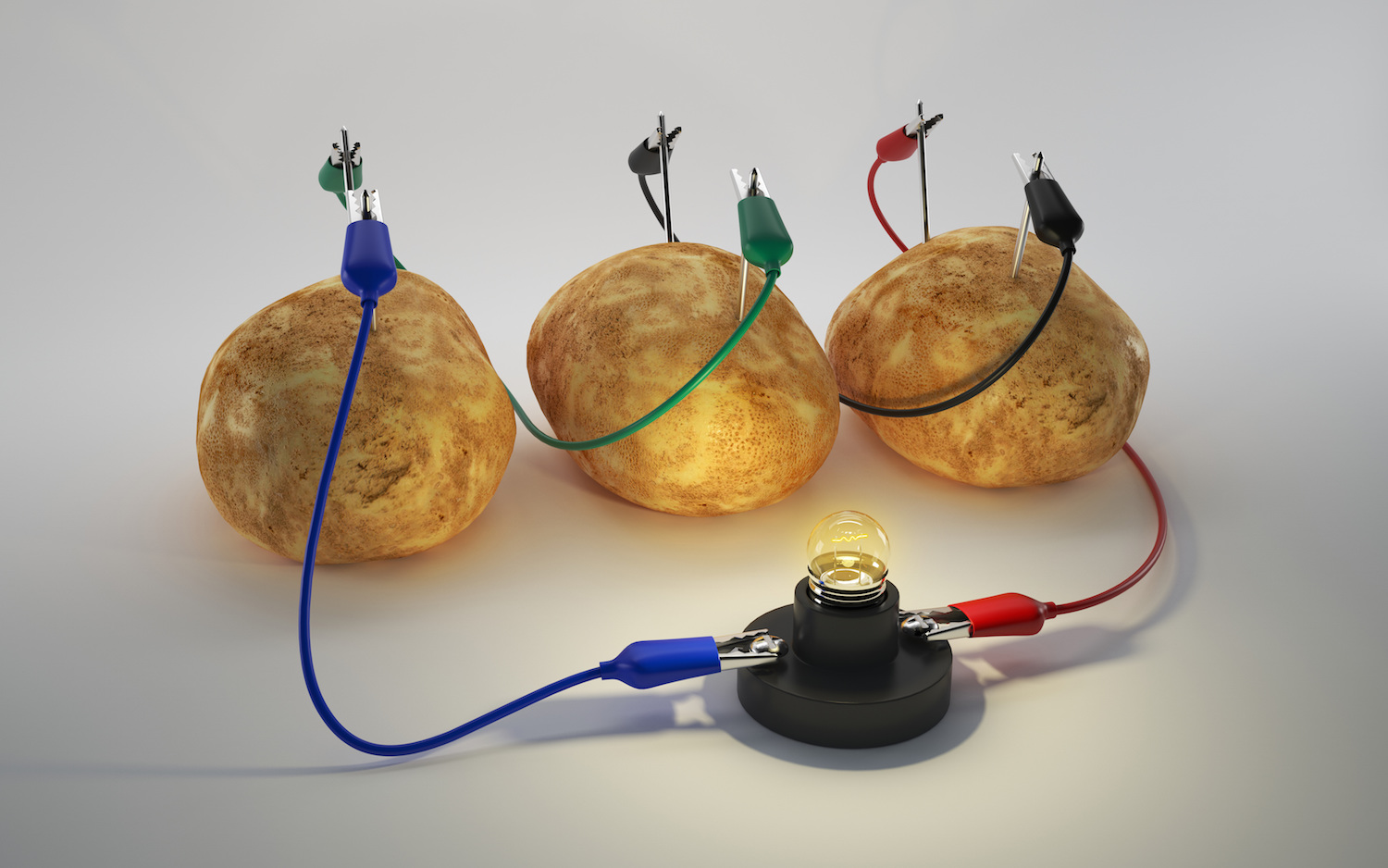 Source: livescience.com
Source: livescience.com
A battery has two terminals one positive and one negative. The acid comes from the citric acid inside each lemon. Powered by Create your own unique website with customizable templates. Batteries are comprised of two different metals suspended in an acidic solution. There are many more types of batteries available on the market now like carbon-zinc cell alkaline cell nickel-cadmium cell Edison cell and mercury cell.
 Source: sciencebuddies.org
Source: sciencebuddies.org
How can a lemon run a clock. The zinc is in the galvanization on the nails and the pennies are actually copper-plated zinc. The apple had the highest voltage measurement at. In my experiment I used copper rod positive end and zinc rod negative end and fruits and vegetables which I had at home and Multimeter to measure the generated electrical energy. In this simple experiment we will be creating our own battery with the use of citrus fruits with a power that is strong enough to make a small bulb light up.
 Source: thoughtco.com
Source: thoughtco.com
Yes you can actually use fruits and vegetables as part of an electric power source. It increased for the apple and the potato. This site is dedicated to such curiosities. A battery has two terminals one positive and one negative. Show full abstract fruit and vegetable waste can be used as a renewable alternative energy source in the form of bio -batteries as a substitute for conventional batteries.
 Source: powerfromearth.blogspot.com
Source: powerfromearth.blogspot.com
Students may insert a small strip of zinc and a small strip of copper into the fruit leaving a gap of about 5cm between the two and making sure they do not come into contact with one another. In my experiment I used copper rod positive end and zinc rod negative end and fruits and vegetables which I had at home and Multimeter to measure the generated electrical energy. Students may insert a small strip of zinc and a small strip of copper into the fruit leaving a gap of about 5cm between the two and making sure they do not come into contact with one another. The voltage varied over time for all test items. How can a lemon run a clock.
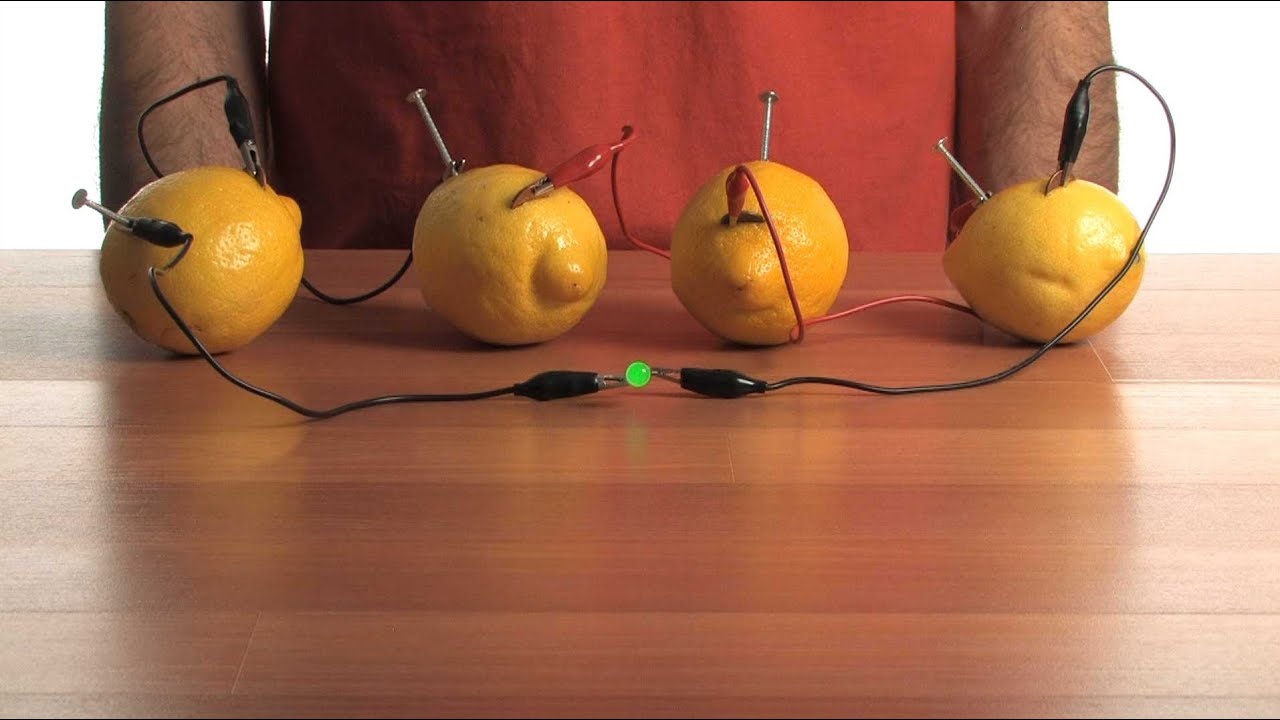 Source: youtube.com
Source: youtube.com
A fruit battery is an example of electrochemistry which forms the basis of all batteries. This site is about electricity. With the Fruit-Power Battery the two metals are zinc and copper. The voltage varied over time for all test items. Typically zinc and copper are common metals.
 Source: youtube.com
Source: youtube.com
Batteries are device that store chemical energy and convert it to electrical energy so using fruit as battery acts like a wet cell that consists of a negative and positive electrode with an electrolyte which conducts ions also copper and zinc metals acts as electrodes whilecitric acid of the fruit is the electrolyte. The zinc is in the galvanization on the nails and the pennies are actually copper-plated zinc. Citrus fruits tend to work well- lemons limes grapefruits and other acidic fruits and vegetables. Batteries are comprised of two different metals suspended in an acidic solution. The acid comes from the citric acid inside each lemon.
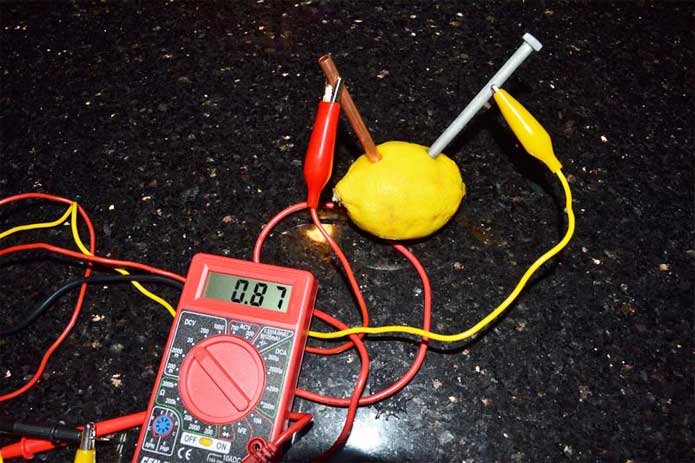 Source: sciencewithkids.com
Source: sciencewithkids.com
It decreased for the onion mango lemon and turnip. With the Fruit-Power Battery the two metals are zinc and copper. It increased for the apple and the potato. How can a lemon run a clock. There are many more types of batteries available on the market now like carbon-zinc cell alkaline cell nickel-cadmium cell Edison cell and mercury cell.
 Source: sciencebuddies.org
Source: sciencebuddies.org
Fruit and Vegetable Batteries By Catherine Clem and Kelly Flora-Brownell Results. A battery has two terminals one positive and one negative. In my experiment I used copper rod positive end and zinc rod negative end and fruits and vegetables which I had at home and Multimeter to measure the generated electrical energy. It increased for the apple and the potato. Yes you can actually use fruits and vegetables as part of an electric power source.
 Source: sciencestruck.com
Source: sciencestruck.com
This site is about electricity. The best food battery is any fruit or vegetable that has high levels of superconductive ions such as potassium or sodium and the proper internal structure to create a working current. Have you ever wondered how a clock will run off of a lemon. In this simple experiment we will be creating our own battery with the use of citrus fruits with a power that is strong enough to make a small bulb light up. The zinc is in the galvanization on the nails and the pennies are actually copper-plated zinc.
If you find this site good, please support us by sharing this posts to your own social media accounts like Facebook, Instagram and so on or you can also save this blog page with the title fruit and vegetable batteries by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.





